Our Pipeline
See how we're shaping the future of precision oncology.
| Program | Target & Molecule | Indication | Isotope | Preclinical | Phase I | Phase IIa | Phase IIb | Notes | |
|---|---|---|---|---|---|---|---|---|---|
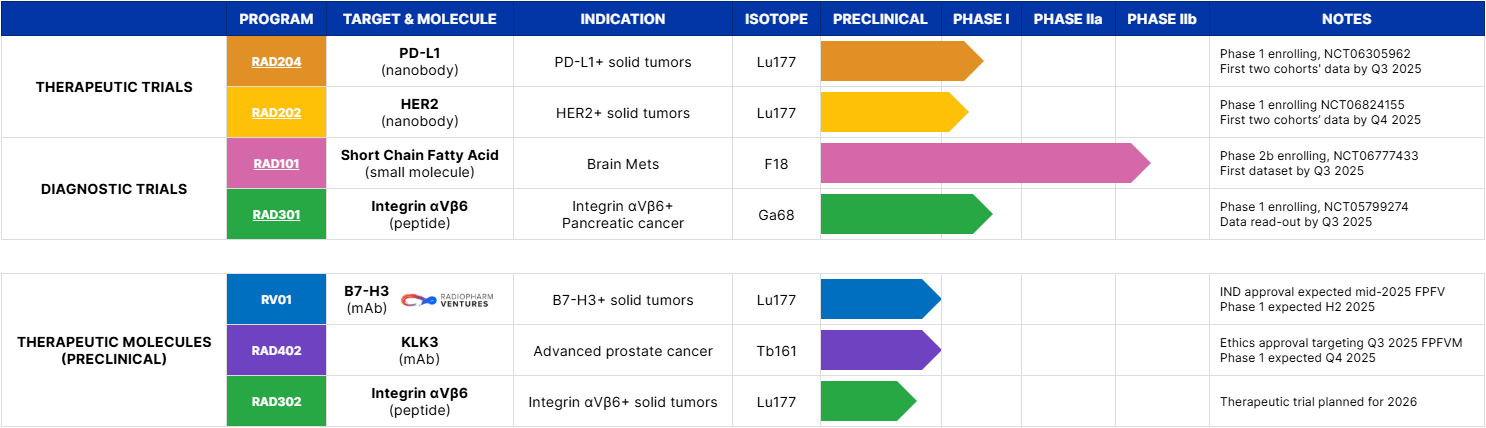
| THERAPEUTIC TRIALS | RAD204 | PD‑L1(nanobody) | PD‑L1+ solid tumors | Lu177 | Phase 1 enrolling, NCT06305962 First two cohorts data by Q3 2025 |
||||
| RAD202 | HER2(nanobody) | HER2+ solid tumors | Lu177 | Phase 1 enrolling NCT06824155 First two cohorts data by Q4 2025 |
|||||
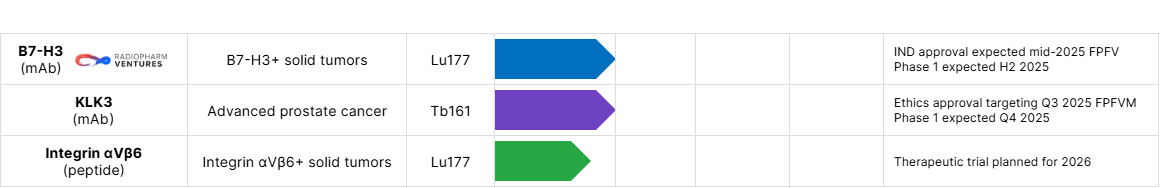
| RV01 | B7‑H3(mAb) | B7‑H3+ solid tumors | Lu177 | Phase 1/2a active, not yet recruiting NCT07189871 FPFV excepted by H2 2025 |
|||||
| DIAGNOSTIC TRIALS | RAD101 | Short Chain Fatty Acid(small molecule) | Brain Mets | F18 | Phase 2b enrolling, NCT06777433 First dataset by Q3 2025 |
||||
| RAD301 | Integrin αvβ6(peptide) | Integrin αvβ6+ Pancreatic cancer | Ga68 | Phase 1 enrolling, NCT05799274 Data read‑out by Q3 2025 |
|||||
| PRECLINICAL | RAD402 | KLK3(mAb) | Advanced prostate cancer | Tb161 | Ethics approval targeting Q3 2025 FPFV Phase 1 expected Q4 2025 |
||||
| RAD302 | Integrin αvβ6(peptide) | Integrin αvβ6+ solid tumors | Lu177 | Therapeutic trial planned for 2026 |
| Program | Target & Molecule | Indication | Isotope | Preclinical | Phase I | Phase IIa | Phase IIb | Notes | |
|---|---|---|---|---|---|---|---|---|---|
| IMAGING TRIAL | RAD101 | Short Chain Fatty Acid(small molecule) | Brain Mets | F18 |
Phase 2b in 5 US centers,
NCT06777433 15 patients dosed / 30 patients total (11/25) Expect to complete enrollment 1Q26 |
||||
| THERAPEUTIC TRIALS | RAD204 | PD-L1(nanobody) | PD-L1+ solid tumors | Lu177 |
Phase 1 in 4 AUS centers,
NCT06305962 DL1 at 30mCi & DL2 at 60mCi completed DL3 at 90mCi recruiting Expect trial completion in 2026 |
||||
| RAD202 | HER2(nanobody) | HER2+ solid tumors | Lu177 |
Phase 1 in 5 AUS centers
NCT06824155 DL1 at 30mCi completed DL2 at 75mCi recruiting Expect trial completion in 2026 |
|||||
| RV01 | B7-H3(mAb) | B7-H3+ solid tumors | Lu177 |
IND approval 07/2025
NCT07189871 Phase 1 in 4 US centers, FPFV expected Q4 2025 First two Dose Levels data in mid-2026 |
|||||
| RAD402 | KLK3(mAb) | Advanced prostate cancer (>90% express KLK3) | Tb161 |
Phase 1 FIH in 4 AUS centers,
NCT07259123 First two Dose Levels data in mid-2026 |
|||||
| IMAGING | RAD301 | Integrin [avB6](peptide) | Integrin αvβ6+ Tumors Pancreatic cancer/ NSCLC | Ga68 |
Phase 1 enrolling
NCT05799274 8 pts dosed / 9 total |
Radiopharm Theranostics’ products are investigational; the safety and effectiveness have not been established.
The investigational products are not approved for sale in any country, for any indication.
More Information
Animal testing is a regulatory requirement to ensure that new pharmaceutical products are safe and effective in humans. At Radiopharm Theranostics, we value the contribution that animals make to science and take animal welfare very seriously. As a result, we only conduct animal testing in countries where the highest animal welfare standards are enshrined in law and where animal welfare and ethical standards in research facilities are constantly monitored by an impartial ethics committee or a relevant authority. Thus, we only work with carefully selected partners in the European Union, USA, UK and Australia.In our research, we are strongly committed to upholding the 4Rs of animal research:
- Replacement: we continuously seek regulatory advice to allow for the substitution of animal studies with in vitro alternatives.
- Reduction: whenever possible, we use novel molecular imaging technology in our animal studies instead of conventional methods and this enables us to reduce the number of animals by 50 to 80%.
- Refinement: we seek to minimise distress to research animals and, as part of our commitment to Replacement, seek to replace the most distressing studies when possible.
- Responsibility: we proudly accept responsibility for animals in our studies and actively engage with our partner organisations conducting research on our behalf.”